Avogadro's number, the number of particles in a mole, can be experimentally determined by first 'counting' the number of atoms in a smaller space and then scaling up to find the number of particles that would have a mass equal to the atomic or molecular mass in grams. Here is some real data from which Avogadro's number can be determined. A mole is defined as the amount of a substance. More specifically, there are 6.02×10 23 particles in a mole of substance. Therefore, if you had 1 mole of feathers and 1 mole of bowling balls, you would have 6.02×10 23 feathers and 6.02×10 23 bowling balls. Now suppose you were asked the question, 'Which weighs more, 100 moles of feathers. The elementary entities that can be represented in moles can be atoms, molecules, monoatomic/polyatomic ions, and other particles (such as electrons). For example, one mole of a pure carbon-12 ( 12 C) sample will have a mass of exactly 12 grams and will contain 6.02214076.10 23 (N A ) number of 12 C atoms. 1 mol = 6.02E23 molecules. Therefore- number of particles in mol of NaCl = 6.02E23 to find mass of a molecule in NaCl take the molar mass of Na and Cl. Add them and you have the molar mass of.
More Chemistry LessonsIn these lessons, we will learn
- how to calculate the mass of a substance when we are given the number of moles (mole to mass conversion).
- how to calculate the number of moles of a substance when we are given the mass (mass to mole conversion).
Mole-Mass Equation
mass = number of moles × molar mass
where mass is in grams and the molar mass is in grams per mole.
Moles to Mass CalculationWe can use the above equation to find the mass of a substance when we are given the number of moles of the substance.
Example:
Calculate the mass of (a) 2 moles and (b) 0.25 moles of iron. (Relative atomic mass: Fe = 56)
Solution:
a) mass of 2 moles of iron
= number of moles × molar mass
= 2 × 56
= 112 g
b) mass of 0.25 mole of iron
= number of moles × molar mass
= 0.25 × 56
= 14 g
Example:
Calculate the mass of (a) 3 moles and (b) 0.2 moles of carbon dioxide gas, CO2. (Relative atomic mass: C = 12; O = 16)
Solution:
a) mass of 1 mole of CO2
= (1 × 12) + (2 × 16)
= 44 g
mass of 3 moles of CO2
= 3 × 44
= 132g
b) mass of 0.2 mole of CO2
= 0.2 × 44
= 8.8 g
Example:
If an experiment calls for 0.200 mol acetic acid (HC2H3O2), how many grams of glacial acetic acid do we need?
Formula: m = nM
- Show Step-by-step Solutions
If an experiment calls for 0.500 mol CaCO3, how many grams of pure calcium carbonate do we need? Mass to Moles Calculation
If we are given the mass of a substance and we are asked to find the number of moles of the substance, we can rewrite the above equation as
Example:
Calculate the number of moles of aluminum present in (a) 108 g and (b) 13.5 g of the element. (Relative atomic mass: Al = 27)
Solution:
a)
b)
Example:
Calculate the number of moles of magnesium oxide, MgO in (a) 80 g and (b) 10 g of the compound. (Relative atomic mass: O = 16, Mg = 24)
Solution:
a) Mass of 1 mole of MgO
= (1 x 24) + (1 x 16)
= 40 g
b)
Examples of mass to mole calculation
Example:
How many moles of acetic acid (HC2H3O2) are present in a 5.00 g sample of pure acetic acid?
- Show Step-by-step Solutions
Examples:

1. How many moles of NAOH are represented by 80.0 grams of NAOH?
2. How many grams will 3.5 moles of NAOH weigh?
Try the free Mathway calculator and problem solver below to practice various math topics. Try the given examples, or type in your own problem and check your answer with the step-by-step explanations.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.
Dalton’s theory that each chemical compound has a particular combination of atoms and that the ratios of the numbers of atoms of the elements present are usually small whole numbers. It also describes the law of multiple proportions, which states that the ratios of the masses of elements that form a series of compounds are small whole numbers. The problem for Dalton and other early chemists was to discover the quantitative relationship between the number of atoms in a chemical substance and its mass. Because the masses of individual atoms are so minuscule (on the order of 10−23 g/atom), chemists do not measure the mass of individual atoms or molecules. In the laboratory, for example, the masses of compounds and elements used by chemists typically range from milligrams to grams, while in industry, chemicals are bought and sold in kilograms and tons. To analyze the transformations that occur between individual atoms or molecules in a chemical reaction, it is therefore essential for chemists to know how many atoms or molecules are contained in a measurable quantity in the laboratory—a given mass of sample.
The Mole: A Chemistry 'Dozen'
Because atoms and molecules are extremely small, there are a great many of them in any macroscopic sample. For example a 1 cm3 of mercury would contain (4.080 times 10^{22}) mercury atoms. The very large numbers involved in counting microscopic particles are inconvenient to think about or to write down. Chemists have chosen to count atoms and molecules using a unit called the mole (mol), from the Latin moles, meaning “pile” or “heap.” One mole is 6.022 x 1023 of the microscopic particles which make up the substance in question. Thus 6.022 x 1023 Br atoms is referred to as 1 mol Br.
The mole is an amount unit similar to familiar units like pair, dozen, gross, etc. It provides a specific measure of the number of atoms or molecules in a bulk sample of matter. The mole provides a link between an easily measured macroscopic property, bulk mass, and an extremely important fundamental property, number of atoms, molecules, and so forth.
The number of entities composing a mole has been experimentally determined to be (6.02214179 times 10^{23}). This is a fundamental constant named Avogadro’s number ((N_A)) or the Avogadro constant in honor of Italian scientist Amedeo Avogadro. This constant is properly reported with an explicit unit of “per mole,” a conveniently rounded version being (6.022 times 10^{23}/ce{mol}).
Using Units of Specific Number of Items is Common
Many familiar items are sold in numerical quantities with distinct names. For example, cans of soda come in a six-pack, eggs are sold by the dozen (12), and pencils often come in a gross (12 dozen, or 144). Sheets of printer paper are packaged in reams of 500, a seemingly large number. Atoms are so small, however, that even 500 atoms are too small to see or measure by most common techniques. Any readily measurable mass of an element or compound contains an extraordinarily large number of atoms, molecules, or ions, so an extremely large numerical unit is needed to count them.
There is a difference in degree, however, because the mole of anything (6.022 x 1023) is so large. A stack of paper containing a mole of sheets would extend more than a million times the distance from the earth to the sun, and 6.022 x 1023 grains of sand would cover all the land in the world to a depth of nearly 2 ft. Obviously there are a great many particles in a mole of anything.
A mole is formally defined as the amount of substance containing the same number of discrete entities (such as atoms, molecules, and ions) as the number of atoms in a sample of pure 12C weighing exactly 12 g. Consistent with this definition, 1 mole of any element contains the same number of atoms as 1 mole of any other element. The masses of 1 mole of different elements, however, are different, since the masses of the individual atoms are drastically different. The molar mass of an element (or compound as we will demonstrate) is the mass in grams of 1 mole of that substance, a property expressed in units of grams per mole (g/mol) (Figure (PageIndex{2})).
Because the definitions of both the mole and the atomic mass unit are based on the same reference substance, 12C, the molar mass of any substance is numerically equivalent to its atomic mass in amu. Per the amu definition, a single 12C atom weighs 12 amu (its atomic mass is 12 amu). According to the definition of the mole, 12 g of 12C contains 1 mole of 12C atoms (its molar mass is 12 g/mol). This relationship holds for all elements, since their atomic masses are measured relative to that of the amu-reference substance, 12C (Table (PageIndex{1})).
| Element | Average Atomic Mass (amu) | Atomic Mass (g/mol) | Atoms/Mole |
|---|---|---|---|
| C | 12.01 | 12.01 | (6.022 times 10^{23}) |
| H | 1.008 | 1.008 | (6.022 times 10^{23}) |
| O | 16.00 | 16.00 | (6.022 times 10^{23}) |
| Na | 22.99 | 22.99 | (6.022 times 10^{23}) |
| Cl | 33.45 | 35.45 | (6.022 times 10^{23}) |
Overview of Origin and Properties of the Mole
The word 'mole' suggests a small, furry burrowing animal to many. But in this lesson, we look at the concept of the mole in chemistry. Learn the incredible magnitude of the mole--and how something so big can help us calculate the tiniest particles in the world.
Converting between Number of Moles and Number of Atoms
Although chemists usually work with moles as units, occasionally it is helpful to refer to the actual number of atoms or molecules involved. When this is done, the symbol (N) is used for the number of species and (n) is used for the number of moles. For example, in referring to 1 mol of helium atoms, we could write
[n_{ce{He}} = 1 text{mol} nonumber]
and
[ N_{ce{He}} = 6.022times 10^{23} nonumber]
Obtaining (N) requires the use of a conversion factor to obtain, which is just (N_A). This which is defined by the equation
[N_{text{A}} = dfrac{N}{n} label{eq1}]
Since for any substance there are 6.022 × 1023 particles per mole, Equation ref{eq1} can be expanded:
[textit{N}_text{A}=dfrac{6.022cdot10^{23}} {1text{ mol}}=6.022times 10^{23}text{ mol}^{text{–1}} label{eq2}]
Equation ref{eq2} is the most important conversion factor in general chemistry. Figure (PageIndex{3}) is a flowchart for converting between the number of moles and the number of atoms. The use of these conversions is illustrated in Example (PageIndex{1}) and Exercise (PageIndex{1}).
Example (PageIndex{1}): Copper Atoms
How many atoms are present in 2.76 mol of copper atoms?
Solution
The definition of a mole is an equality that can be used to construct a conversion factor.
[2.76, cancel{mol, ce{Cu}}times frac{6.022times 10^{23}atoms, ce{Cu}}{cancel{mol, ce{Cu}}}=1.66times 10^{24} text{molecules} , ce{Cu} nonumber]
Exercise (PageIndex{1})
How many molecules are present in (4.61 times 10^{-2}) mol of helium atoms?
2.78 × 1022 molecules
Converting between Mass and Number of Moles
The molar mass of a substance is defined as the mass in grams of 1 mole of that substance. One mole of isotopically pure carbon-12 has a mass of 12 g. For an element, the molar mass is the mass of 1 mol of atoms of that element. That is, the molar mass of a substance is the mass (in grams per mole) of 6.022 × 1023 atoms of that substance. In each case, the number of grams in 1 mol is the same as the number of atomic mass units that describe the atomic mass.
The periodic table lists the atomic mass of carbon as 12.011 amu; the average molar mass of carbon—the mass of 6.022 × 1023 carbon atoms—is therefore 12.011 g/mol.
Reminder: Molar Masses (Atomic Masses) are Weighted Averages of Isotopic Masses Ncr printers driver.
As discussed in Section 1.9, The molar mass of naturally-occurring carbon is different from that of carbon-12 isotope because carbon occurs as a mixture of carbon-12, carbon-13, and carbon-14. One mole of carbon still has 6.022 × 1023 carbon atoms, but 98.89% of those atoms are carbon-12, 1.11% are carbon-13, and a trace (about 1 atom in 1012) are carbon-14. Similarly, the molar mass of uranium is 238.03 g/mol.
The mole is the basis of quantitative chemistry and provides chemists with a way to convert easily between the mass of a substance and the number of individual atoms, molecules, or formula units of that substance. Conversely, it enables chemists to calculate the mass of a substance needed to obtain a desired number of atoms. For example, to convert moles of a substance to mass, the following relationship is used: Logitech mice & touchpads driver.
[(text{moles}) times (text{molar mass}) rightarrow text{mass} label{3.4.1}]
or, more specifically,
[ cancel{text{moles}} times left ( {text{grams} over cancel{text{mole}} } right ) = text{grams} nonumber ]
Conversely, to convert the mass of a substance to moles:
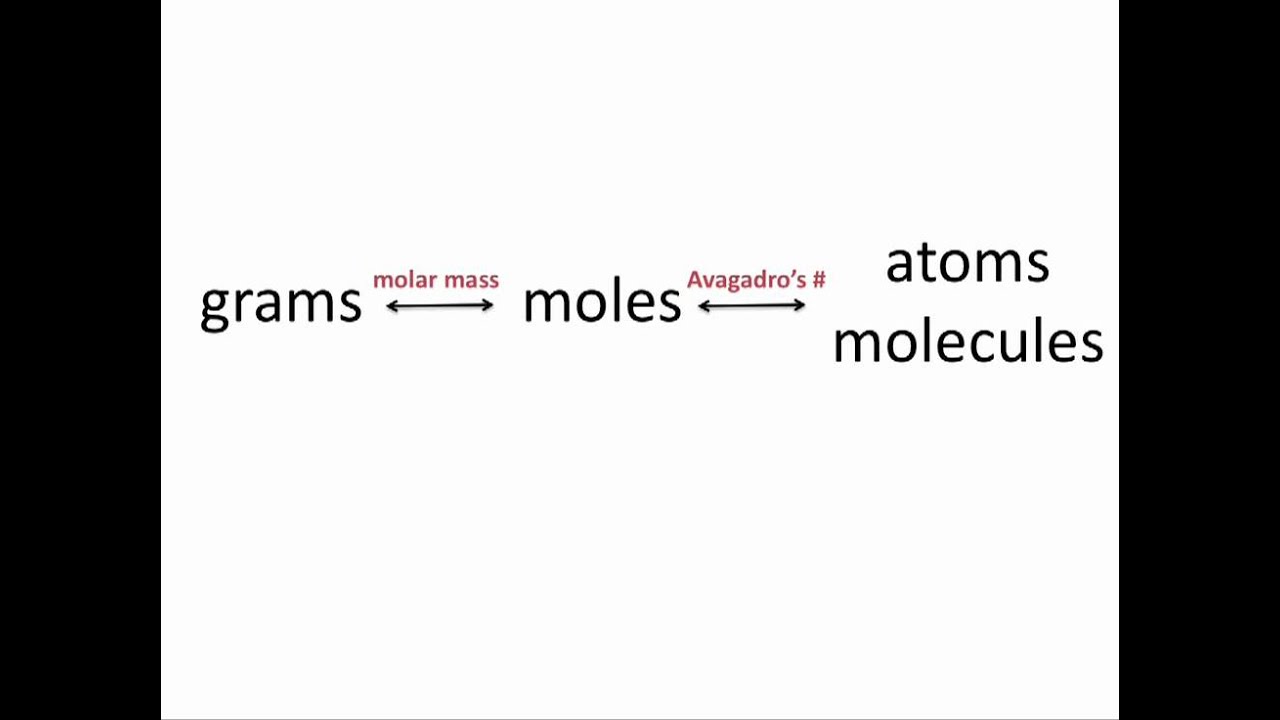
[ left ( {text{grams} over text{molar mass} } right ) rightarrow text{moles} label{3.4.2A}]
[ left ( { text{grams} over text{grams/mole}} right ) = cancel{text{grams}} left ( {text{mole} over cancel{text{grams}} } right ) = text{moles} label{3.4.2B}]
If know the mass and chemical composition of a substance, we can determine the number of moles and calculate number of atoms or molecules in the sample. Likewise, if we know the number of moles of a substance, we can derive the number of atoms or molecules and calculate the substance’s mass.
Particles In Moles Calculator
Example (PageIndex{2}): Deriving Moles from Grams for an Element
According to nutritional guidelines from the US Department of Agriculture, the estimated average requirement for dietary potassium is 4.7 g. What is the estimated average requirement of potassium in moles?
Solution
The mass of K is provided, and the corresponding amount of K in moles is requested. Referring to the periodic table, the atomic mass of K is 39.10 amu, and so its molar mass is 39.10 g/mol. The given mass of K (4.7 g) is a bit more than one-tenth the molar mass (39.10 g), so a reasonable “ballpark” estimate of the number of moles would be slightly greater than 0.1 mol.
The molar amount of a substance may be calculated by dividing its mass (g) by its molar mass (g/mol):
The factor-label method supports this mathematical approach since the unit “g” cancels and the answer has units of “mol:”

[ mathrm{4.7; cancel{g} K left ( dfrac{mol; K}{39.10;cancel{g}}right)=0.12;mol; K} nonumber]
The calculated magnitude (0.12 mol K) is consistent with our ballpark expectation, since it is a bit greater than 0.1 mol.
Exercise (PageIndex{2}): Beryllium
Beryllium is a light metal used to fabricate transparent X-ray windows for medical imaging instruments. How many moles of Be are in a thin-foil window weighing 3.24 g?
0.360 mol
Example (PageIndex{3}): Deriving Grams from Moles for an Element
A liter of air contains (9.2 times 10^{−4}) mol argon. What is the mass of Ar in a liter of air?
Solution
The molar amount of Ar is provided and must be used to derive the corresponding mass in grams. Since the amount of Ar is less than 1 mole, the mass will be less than the mass of 1 mole of Ar, approximately 40 g. The molar amount in question is approximately one-one thousandth (~10−3) of a mole, and so the corresponding mass should be roughly one-one thousandth of the molar mass (~0.04 g):
In this case, logic dictates (and the factor-label method supports) multiplying the provided amount (mol) by the molar mass (g/mol):
[mathrm{9.2 times10^{-4}; cancel{mol} ; Ar left( dfrac{39.95;g}{cancel{mol};Ar} right)=0.037;g; Ar} nonumber]
The result is in agreement with our expectations, around 0.04 g Ar.
Exercise (PageIndex{3})
What is the mass of 2.561 mol of gold?
504.4 g
Example (PageIndex{4}): Deriving Number of Atoms from Mass for an Element
Copper is commonly used to fabricate electrical wire (Figure (PageIndex{6})). How many copper atoms are in 5.00 g of copper wire?
Solution
The number of Cu atoms in the wire may be conveniently derived from its mass by a two-step computation: first calculating the molar amount of Cu, and then using Avogadro’s number (NA) to convert this molar amount to number of Cu atoms:
Considering that the provided sample mass (5.00 g) is a little less than one-tenth the mass of 1 mole of Cu (~64 g), a reasonable estimate for the number of atoms in the sample would be on the order of one-tenth NA, or approximately 1022 Cu atoms. Carrying out the two-step computation yields:
[mathrm{5.00:cancel{g}:Culeft(dfrac{cancel{mol}:Cu}{63.55:cancel{g}}right)left(dfrac{6.022times10^{23}:atoms}{cancel{mol}}right)=4.74times10^{22}:atoms: of: copper}]
The factor-label method yields the desired cancellation of units, and the computed result is on the order of 1022 as expected.
Exercise (PageIndex{4})
The Number Of Particles In A Mole Is Known As
A prospector panning for gold in a river collects 15.00 g of pure gold. How many Au atoms are in this quantity of gold?
(4.586 times 10^{22}; Au) atoms
Contributors and Attributions
What Is A Mole Unit
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd..a7ac8df6@9.110).
