- Chlorine Valence Electrons Gain Or Lose
- Chlorine Valence Electrons Atom
- What Is Chlorine Valence Electrons
- Chlorine Valence Electrons Losing
- Valence Electron Groups
How many valence electrons are in an atom of chlorine?

- There are two ways to find the number of valence electrons in Chlorine (Cl). The first is to use the Periodic Table to figure out how many electrons Chlorine.
- The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z. Electron configuration of Chlorine is Ne 3s2 3p5.
- The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Chlorine is 17. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
The 5 Cl atoms contribute 5 electrons, one for each atom. This makes the valence shell electrons 10. Total valence shell electron pairs are 5. The PCl5 structure has 2 different kinds of P-Cl bonds. All the Phosphorus-Chlorine equatorial bonds make 90 degrees and 120 degrees bond angles, two each, with the further bonds in the atom.

3 Answers
Explanation:
The electron configuration of chlorine is
The
Chlorine Valence Electrons Gain Or Lose
In a picture, the valence electrons are the ones in the outermost shell.
You can see in the diagram below that there are seven electrons in the outermost circle.
Chlorine Valence Electrons Atom
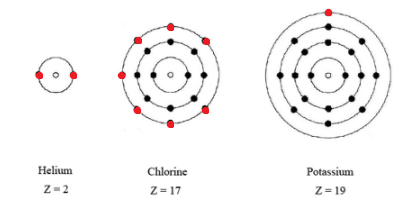

Additionally, a more basic way of determining the number of valence electrons would be to simply look at what group Cl is in.
It is in Group 17, which means it has 7 valence electrons.
Chlorine has an atomic number 17. Atomic number is the number of protons present in the nucleus of an atom. All the atoms of an element have same atomic number. In every stable atom the number of electrons is equal to the number of protons. Therefore, the number of electrons is equal to the number of protons in an chlorine atom. This means, that the number of electrons present in an chlorine atom is 17.
The electronic configuration of chlorine atom is:-
E.C - K L M
2 8 7
Where k, l, ,m are the shells/orbits/ energy levels of an chlorine atom.
What Is Chlorine Valence Electrons
Valence electrons are the number of electrons present in the outermost shell of an atom. Now, the last shell of chlorine atom has 7 electrons in it. Therefore, there are 7 valence electrons present in an chlorine atom.
Here, stable means that atom has not formed a ion yet.
Chlorine has seven valence electrons.
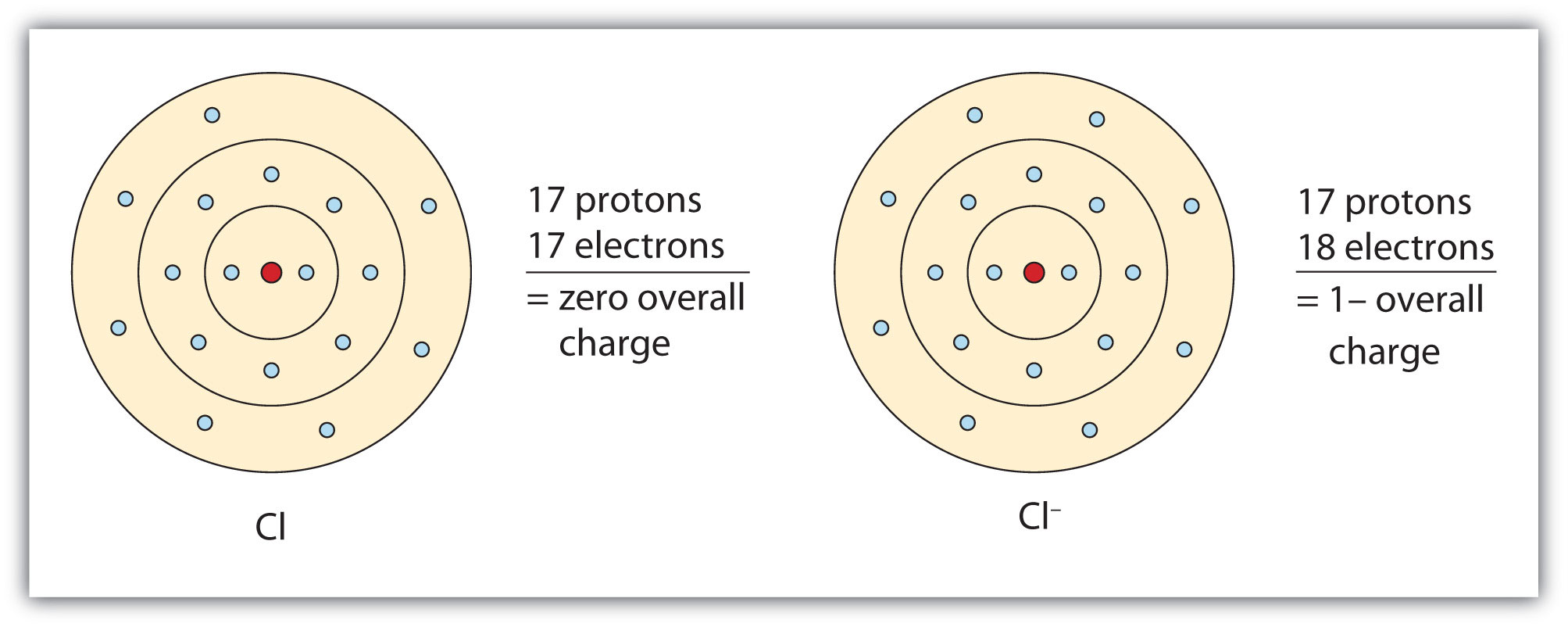
Chlorine has atomic number 17. Epson laptops & desktops driver download for windows 10.
Chlorine Valence Electrons Losing
The atomic number is the number of protons present in the nucleus of an atom.All the atoms of an element have same atomic number.
In every stable atom the number of electrons is equal to the number of protons. Stable means that atom has not formed a ion yet.
Therefore, the number of electrons is equal to the number of protons in an chlorine atom.
Valence Electron Groups
This means, that the number of electrons present in an chlorine atom is 17.
The energy shells of an atom are in the order K, L, M …
Dis-transics driver download. So the electronic configuration of chlorine atom is: K, L, M = 2, 8, 7
Valence electrons are the number of electrons present in the outermost shell of an atom.
The last shell of a chlorine atom has 7 electrons in it.
Drivers marvell bluetooth devices. Therefore, there are 7 valence electrons in an chlorine atom.
Here is a video which gives a quick discussion of how to determine how many valence electrons atoms of different elements have.
Video from: Noel Pauller
Related questions
